The literature search is a key part of the clinical evaluation. It usually involves numerous hours of work. This article will give you six tips to help you efficiently carry out and fully document the literature search.
In the literature search, manufacturers gather together scientific articles, among other reasons, to document the state of the art and provide evidence of the safety, performance, and clinical benefit of their device. But more on this later.
There are now several MDCG documents available on the topic of the clinical evaluation, but none that describe how to carry out and document the literature search for the clinical evaluation.
MEDDEV 2.7/1 is also the most important guideline for the literature search under the MDR. The Medical Device Coordination Group says the same.
“For general guidance on a literature search, see MEDDEV 2.7/1 Revision 4, A5. Literature search and literature review protocol, key elements”
Section D, MDCG 2020-13
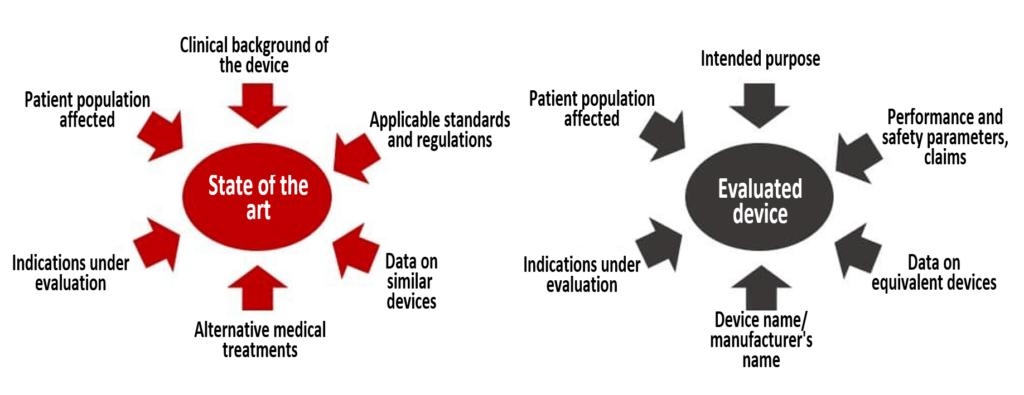
Through the literature search, you find literature on the device under evaluation, the equivalent device and the state of the art, including alternative examination and treatment methods.
Annex 5 of MEDDEV 2.7/1 Revision 4 describes the most important aspects to remember when documenting the literature search. In it, the guideline requires the objective of the literature search(es) to be documented. Examples of such objectives are:
Manufacturers also have to document the search methods.
There will be more on documentation in the second tip.
Additional informationAdditional information can be found in our in-depth article on MEDDEV 2.7/1.
The MDCG documents do not currently offer any concrete guidance on how the literature search should be carried out. The MDCG document 2020-13 “Clinical evaluation assessment report template” is nevertheless useful:
It is primarily aimed at clinical evaluation reviewers, particularly notified bodies, but it also provides indirect guidance for anyone carrying out a clinical evaluation. Section D deals with literature search and literature review. The requirements listed in this section are the same as the ones in MEDDEV 2.7/1 Revision 4. The focus is on:
The MDCG 2020-13 document refers to MEDDEV 2.7/1 Revision 4. So, save some time for the MDCG document and be glad that you can continue working with MEDDEV 2.7/1 Revision 4, especially when it comes to the literature search.
Other guidance documents on the preparation of the clinical evaluation include, for example, IMDRF MDCE WG/N57FINAL:2019.
Incomplete documentation of the literature search will result in a non-conformity. This can lead to unnecessary queries or even deviations in the audit, since the MDCG document 2020-13 explicitly requires notified bodies to review the literature search documentation. It requires reviews to evaluate the following metadata:
These metadata are incorporated into the literature search protocol.
MDCG 2020-13 requires auditors to pay special attention to the exclusion criteria.
The clinical evaluation should clearly describe the selection criteria with respect to the regulatory purpose to which it will apply. The CER should clearly differentiate between the two types of data (device under evaluation or an equivalent device, state of the art or alternative treatment option). If the data does not relate to either of the above, provide a rationale with respect to its inclusion.
Section D, MDCG 2020-13
MDCG 2020-13 requires manufacturers to define and document the selection criteria for the literature searches. The selection criteria should be defined in the context of the clinical evaluation and distinguish between at least two searches for data or information:
You can find out more on these two searches in tip 3.
The complete documentation of the literature search doesn’t just include the literature search protocol.
The documentation includes all the following documents:
Most clinical evaluations that the Johner Institute receives for revision have been rejected by notified bodies because of literature searches where the above were not, or not completely, documented and available.
You are not free to choose what to look for. MEDDEV 2.7/1 Revision 4 requires your literature search to cover at least two essential topics:
Different search terms are used depending on the objective (see Fig. 1).

You can’t neglect any of these searches. Otherwise there is a risk of a non-conformity in the audit.
The PICO method will help you with literature searches, for example, when trying to find search criteria for the state of the art.
Patient/Population; Intervention; Comparison; Outcome
The PICO method is used in evidence-based medicine in particular and recommended for use in clinical evaluation literature searches by MEDDEV 2.7/1 Revision 4.
Just as important as the search strategy is choosing which databases you are going to search. The MDR does not give any concrete advice on how to choose the literature databases. However, in article 2(48) it requires peer-reviewed publications.
With a few exceptions, PubMed only contains peer-reviewed publications. In contrast to Embase, PubMed allows free search and registration. However, access to the full texts is not always free of charge on PubMed.
In addition, the MDR mentions “relevant specialist literature” or “databases”. However, the EU regulation leaves it up to the authors of the clinical evaluation to decide which databases they search.
In section D, the MDCG 2020-13 states that multiple databases should be used to minimize bias in the literature review.
MEDDEV 2.7/1 Revision 4, Annex 4 provides some guidance for selecting suitable literature databases. It recommends using MEDLINE, PubMed and other databases such as EMBASE or the Cochrane CENTRAL trials register but does not explicitly require them.
| Database | Benefits according to MEDDEV 2.7/1 Revision 4, Annex 4 |
| PubMed/Medline | Good starting point for a search. Completeness cannot be guaranteed (possibly incomplete coverage of European journals) |
| EMBASE/Excerpta Medica | Adequate coverage of medical devices and therapies used in Europe. Facilitate searches by device name and manufacturer |
| Cochrane CENTRAL trials register | Same as EMBASE |
So, you can save costs and start the search in PubMed and use additional databases (e.g., EMBASE) to cover European topics (therapies or medical devices in use in Europe).
A further list of possible sources for literature and clinical data can be found in the article on clinical data.
The use of (Boolean) operators allows you to narrow down your literature search, which saves you the trouble of reading non-specific literature sources. However, you can also use Boolean operators to expand the search, especially if you don’t find enough literature sources.
The operators can be used to combine different search terms according to the context. The most well-known Boolean operators are AND, OR and NOT.
Quotation marks and round brackets are also useful for improving the quality of the search results. Use them to get relevant and specific search results.
The following table illustrates this with some examples:
| Search term | Number of publications found |
| Ice pack | 1154 |
| “Ice pack” | 244 |
| “Ice pack” AND “reduction of pain” OR “reduction of edema” | 181,916 |
| “Ice pack” AND (“reduction of pain” OR “reduction of edema”) | 19 |
Note that the different databases use different operators. Check the database’s website to find out more. This also affects the interpretation of the search phrase when no brackets are used, as shown in the last example in the previous table. Some databases interpret this search phrase as (“ice pack” AND “reduction of pain”) OR “reduction of edema”.
The literature search often returns several hundred publications. At this point, you may wonder whether it is necessary to read the full text of each publication.
You (still) don’t have to read the full texts of all the documents found in the initial search. You can exclude obviously non-relevant publications on the basis of the abstract if they are not related to the clinical evaluation and, for example, don’t have anything to do with the device under evaluation.
However, you should read the full text publication during the literature review at the latest. The MDCG agrees with this view:
“Abstracts lack sufficient detail to allow issues to be evaluated thoroughly and independently, but may be sufficient to allow a first evaluation of the relevance of a paper. Copies of the full text papers and documents should be obtained for the appraisal stage.”
Section D, MDCG 2020-13
You should continue to use MEDDEV 2.7/1 Revision 4 as a guide for the literature search. The MDCG 2020-13 document refers to the requirements of MEDDEV 2.7/1 Revision 4 and encourages clinical evaluation reviewers to pay close attention to the following topics:
Control the range and specificity of the publications found in your literature search by using (Boolean) operators. Save time by excluding obviously irrelevant publications at an early stage, particularly if they do not relate to the state of the art or the device under evaluation.
Remember that in addition to the state of the art, you must at least research the information on equivalent devices. In other words, you must carry out at least two searches.
If you use the tips in this article, you will comply with the MDR’s requirements for literature searches.
Do you have any questions about implementation in specific cases? Email us or send us a contact request. We can review and trim down your documents to ensure conformity so that your clinical evaluation passes the audit.